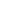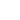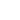2.2.4.2 Van der Waals probe-sample attraction
As it is shown in the chapter on Van der Waals (VdW) forces, the potential of the molecules pairwise interaction depends on the distance as
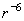 . The corresponding force is equal to its derivative with respect to distance
. The corresponding force is equal to its derivative with respect to distance
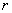 :
:
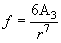
(1)
where
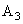 – Hamaker constant.
– Hamaker constant.
Basing on this microscopic description we can determine the attraction force between the probe and the whole sample. It is equal to the sum of all pairwise interactions between cantilever molecules and the studied surface:
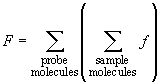
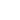
(2)
It is clear that the result will depend sufficiently on the problem geometry.
Ignoring the discrete distribution of interacting centers (molecules) one can easily proceed from the pair summation (2) to the double integral:
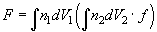
(3)
where
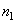 and
and
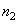 – concentrations of tip and sample molecules (density).
– concentrations of tip and sample molecules (density).
Let us calculate the inner integral denoting it as
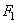 . Its physical meaning is the interaction force between a single molecule and a plane. The attraction force (1) decays sharply with distance (
. Its physical meaning is the interaction force between a single molecule and a plane. The attraction force (1) decays sharply with distance (
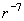 ) therefore, the distant parts of the system do not contribute substantially into the integral. Thanks to this, the integration can be performed over the whole half-space as if it was a uniform sample.
) therefore, the distant parts of the system do not contribute substantially into the integral. Thanks to this, the integration can be performed over the whole half-space as if it was a uniform sample.
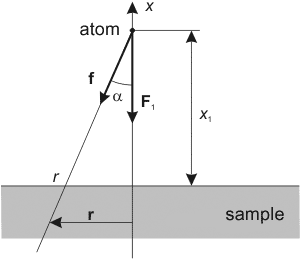
Fig. 1. The single atom – flat sample system.
Introducing the cylindrical coordinates as shown on the Fig. 1, consider the origin of coordinates to be our molecule. From the problem symmetry it is clear that the resulting force is directed downward vertically. In this case the horizontal components of the attraction force between a molecule and two symmetrical with respect to the
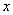 -axis molecules are compensated. That is why it is convenient to consider only vertical force component:
-axis molecules are compensated. That is why it is convenient to consider only vertical force component:
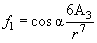
(4)
This force is the same for all points of the ring with radius
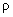 , so integration over the angle around the
, so integration over the angle around the
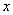 -axis just comes to multiplication by
-axis just comes to multiplication by
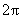 . Further calculations are easy so we get:
. Further calculations are easy so we get:
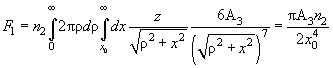
(5)
To take the outer integral in (3) we need to integrate over the tip volume:
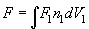
(6)
Therefore, further calculations should be performed for the specific cantilever tip (see Appendices).
Summary.
- To find the probe-sample interaction force one needs to integrate the pairwise Van der Waals interaction of cantilever and sample molecules.
- The force of Van der Waals interaction between a separate molecule and a flat sample decreases with a distance as
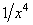 .
. - The probe-sample interaction force can be found by integrating the attraction of all molecules to the sample surface.