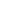AFM Spectroscopies
SFM can be used not only as tool for topography acquisition but also can be used to produce spatially resolved maps of the surface or material properties of a sample; these include charge density, adhesion and stiffness, as well as the force required to break specific igand–receptor bonds. SFM can also be used as a tool for force spectroscopy – measuring forces as a function of distance. For oscillating cantilever tip-sample force can affect some other characteristics of cantilever oscillation – amplitude, frequency, phase, dissipation etc. Correspondingly dependence of these characteristics upon tip-sample distance can also be regarded as spectroscopic data.
Force is measured in an SFM by collecting a force curve, which is a plot of cantilever deflection, dc, as a function of sample position along the z-axis (i.e. towards or away from the probe tip; the z-piezo position). It assumes a simple relationship (i.e. Hooke’s Law) between the force, F, and the cantilever deflection:
F = - k dc
where k is the spring constant of the cantilever. Some other forces included in tip-sample interaction under dc approach or retracting motion are presented on the figure left [1]. Used definitions see below.
The interpretation of AFM force curves relies almost entirely on established force laws, particularly those determined using the SFA [2]. These force laws describe force as a function of the probe–sample separation distance (D) rather than as a function of the z-piezo position. Thus, to be useful, the force curves must be transformed into descriptions of force as a function of distance, F(D). However, current SFMs do not have an independent measure of D. Instead, the transformation to D is achieved by subtracting the cantilever deflection from the z-piezo movement.
For a very hard surface, zero separation is defined as the region in the force curve in which the cantilever deflection is coupled 1:1 with the sample movement; this appears in the force curve as a straight line of unit slope. A corrected curve is called a force–distance curve. Notice that determining D by this approach requires that the tip make contact with the sample.
In practice, there are two factors (long-range forces and sample elasticity) that can make determining the point of contact very difficult. A complete force curve includes the forces measured as the probe approaches the sample and is retracted to its starting position. Because the forces on the tip can vary as it is moved toward or away from the sample, for the purposes of presentation, we will divide the force curve into approach and retraction portions and consider them separately.
References
- TIBTECH 17, 143 (1999).
- Israelashvili, J.N. (1992) Intermolecular and Surface Forces, Academic Press.
Definitions
A Hamaker constant
a Monomer length
D Probe–sample separation distance
E Elastic modulus
k Boltzmann’s constant
L Brush thickness in a good solvent
L* Inverse Langevin function
N Number of units in polymer
R Radius of probe sphere
s Mean distance between polymers
T Absolute temperature
U Bond energy
x Elongation of polymer
δ Indentation depth
ε Dielectric of the medium
γ Surface energy between tip and sample
γL Surface energy of the liquid
ν Poisson ratio
Λ Characteristic length of bond
λ Debye length of the medium
θ Angle related to the geometry of the tip–sample contact
σR Surface-charge density of sphere
σS Surface-charge density of sample
τ Period over which the bond will rupture
τ0 Reciprocal of the natural bond frequency