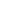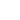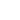3.2 Confocal Microscopy
Introduction.
A confocal microscope differs from a "classical" optical microscope (see chapter 3.1) in the fact that every moment of time there is formed an image of one object point while a whole image is assembled by scanning (moving a specimen or readjusting an optical system). In order to register light from only one point, a pinhole aperture is situated behind the objective lens so that the light emitted by the studied point (red rays in Fig. 1b) passes through the aperture and is detected while light from the other points (e.g. blue rays in Fig. 1b) is at most excluded. The second feature is that the illuminator produces not the uniform lighting of the field of view but focuses light into the studied point (Fig. 1c). This can be done by placing a second focusing system behind a specimen; in this case, however, the specimen should be transparent. Moreover, the objective lenses are usually expensive enough, so utilization of a second focusing system for illumination is of little preference. An alternative is the use of a beam splitter for the purpose of incident and reflected light could be focused by the same objective (Fig. 1d). Besides, such arrangement facilitates system adjustment.
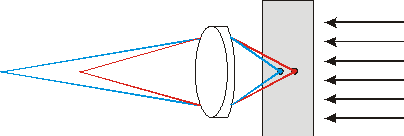
Fig. 1a. Traces of light rays in conventional microscope. The photodetector receives light from various points of a specimen.
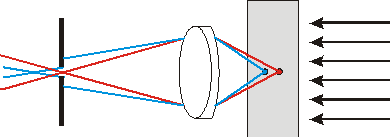
Fig. 1b. Aperture utilization allows to reduce sufficiently background illumination from specimen points beyond the studied area.
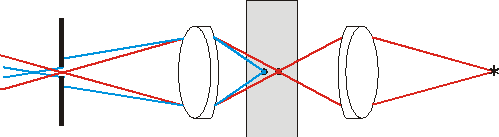
Fig. 1c. Additional contrast increase is due to illumination light focusing into the analyzable point.
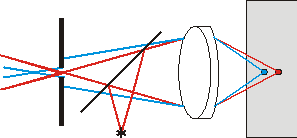
Fig. 1d. Arrangement with a beam splitter simplifies the microscope construction and facilitates its adjustment due to the objective two-fold use
(for illumination and reflected light collection).
It is clear that the application of the confocal scheme should increase the image contrast because "stray" light from points adjacent to the studied one does not enter the detector. Note that the contrast increase is achieved at the expense of complicated scan by specimen or by light beam systems utilization. Detailed examination of existing confocal microscope designs is beyond the scope of this chapter. Further information on the matter can be found in reviews [1-11].
Resolution and contrast in confocal microscopy.
Now let us examine mathematically how and how much the contrast is changed when utilizing the confocal microscopy. Firstly, because the light in the confocal microscope passes through the objective twice, the point spreading function (designated further as PSF, see definition in chapter 3.1) is given by
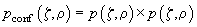
(1)
For the sake of convenience each PSF will be qualified as a probability of a photon hitting the point with coordinates
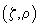 or a photon detection from the point with coordinates
or a photon detection from the point with coordinates
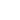
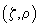 ; then the confocal PSF is a product of independent probabilities. Fig. 2 shows a representation of conventional and confocal PSF.
; then the confocal PSF is a product of independent probabilities. Fig. 2 shows a representation of conventional and confocal PSF.
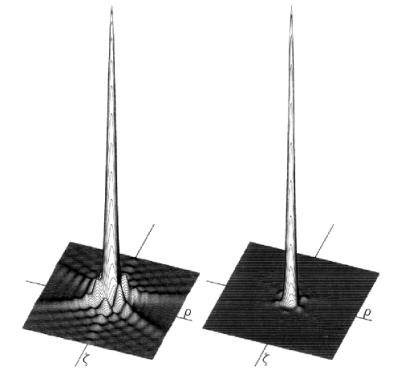
Fig. 2. Confocal PSF
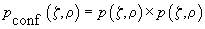 is shown on the right,
is shown on the right,
conventional PSF
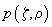 – on the left.
– on the left.
If we use the Rayleigh criterion for the resolution (26% dip of the maximum intensity), the result is a slight increase in resolution for the confocal microscope:
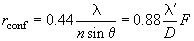
(2)
as compared with the conventional optical microscope
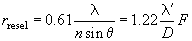
(3)
where 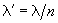 .
.
However, the major advantage of a confocal microscope is a sufficient increase in the contrast rather than resolution improvement in accordance with the Rayleigh criterion. In particular, the relation of the first ring maximum amplitude to the amplitude in the center is 2% in case of conventional PSF in a focal plane while in case of a confocal microscope this relation is 0.04%. The practical importance of this factor is illustrated in Fig. 3. From the top part of the picture it can be seen that a dim object (intensity 200 times less than of a bright one) can not be detected in conventional microscope though the separation distance between objects exceeds that of the Rayleigh criterion. In a confocal microscope (bottom part of Fig. 3) this object should be well registered.
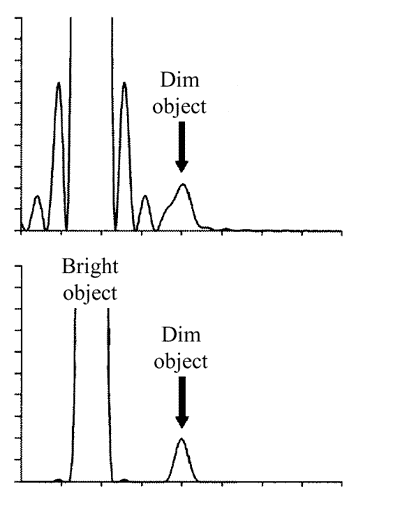
Fig. 3. Intensity profiles for conventional (top picture) and confocal (bottom picture) microscopes.
Intensity maximum of the dim object is 200 times less than that of the bright one.
The intensity distribution along the optical axis in a confocal microscope is given by the following expression:
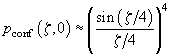
(4)
Then, using the Rayleigh criterion for the resolution in the direction along the optical axis we can write:
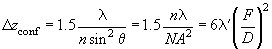
(5)
Notice that one should distinguish this resolution and depth of focus in a conventional microscope. Generally, the depth of focus is hundreds times more than the resolution along the optical axis.
The effect of an aperture in a focal plane.
One of parameters, that was not taken into consideration above, is the size of an aperture in a focal plane of illuminating and collecting lenses. Notice that PSF for conventional and confocal microscopes was calculated under assumption that a source is point-like. Therefore the obtained PSF describe properties of an objective lens and the aperture image in the object plane determines areas whose light is registered by the photodetector. Lowering the aperture size obviously decreases the amount of the passing light, increases noise level and, finally, can reduce all advantages in the contrast to nothing. Thus, the problem of choosing the aperture optimal size and reasonable compromise is quite relevant.
The use of aperture with the size which is less than that of the Airy disk just results in intensity loss and do not affect resolution. If aperture size is that of the Airy disk, the objective lens resolution is maximal. The best compromise, however, is the aperture size which is 3-5 times more than that of the Airy disk. The size considered here should be understood as the image size in the object plane, therefore, the actual aperture size depends on the lens magnification. In particular, when lens with 100x magnification is used, the 1 mm orifice of the diaphragm is projected onto the object plane as a circle with radius 10 micron.
In order to consider mathematically the presence of an aperture and to obtain a new function of intensity distribution one should perform convolution:
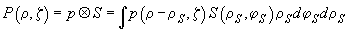
(6)
and for a confocal microscope to multiply the obtained function
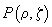 by
by
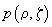 . The resulting intensity distribution in case of the aperture size of 5 Airy disks is shown in Fig. 4.
. The resulting intensity distribution in case of the aperture size of 5 Airy disks is shown in Fig. 4.
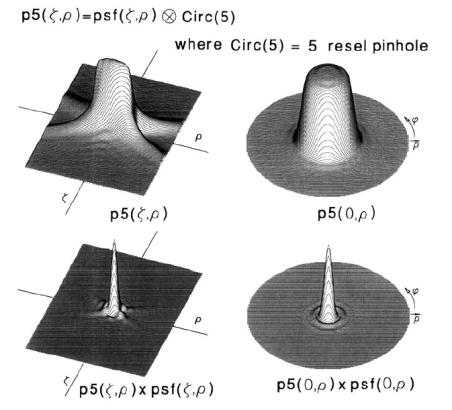
Fig. 4. Point spreading functions for conventional microscope with an aperture size of
5 Airy disks (top pictures) and for confocal microscope (bottom pictures).
Summary.
- Confocal microscopy provides an image contrast increase due to the studied area illumination via focusing objective lens and an aperture placement in the image plane before the photodetector. Such contrast improvement allows for resolving objects having intensity difference up to 200:1.
- In confocal microscopy, the resolution in the object plane is slightly increased (1.5 times) while the resolution along the optical axis is high.
- These improvements are obtained at the expense of the utilization of mechanisms for scanning either by moving a specimen or by readjustment of an optical system. Scanning application allows to increase field of view as compared with conventional microscopes.
References.
- Robert H. Webb "Confocal optical microscopy" Rep. Prog. Phys. 59 (1996) 427-471.
- Richards B. and Wolf E. "Electromagnetic diffraction in optical systems II. Structure of the image field in an aplanatic system" Proc. R. Soc. A 253 (1959) 358-379.
- Kino G. S. and Corle T. R., 1989 Confocal scanning optical microscopy Phys. Today 42 55–62.
- Pawley 1991 J B Fundamental and practical limits in confocal light microscopy Scanning 13 184–98.
- Shotton D., (ed) 1993 Electronic Light Microscopy—Techniques in Modern Biomedical Microscopy (Wiley-Liss) p. 351.
- Slater E. M. and Slater H. S., 1993 Light and Electron Microscopy (Cambridge: Cambridge University Press).
- Stevens J. K., Mills L. R. and Trogadis J. (eds) 1993 Three-Dimensional Confocal Microscopy (San Diego, CA: Academic).
- Webb R. H., 1991 Confocal microscopes Opt. Photon. News 2 8–13.
- Wilson T. 1985 Scanning optical microscopy Scanning 7 79–87.
- Wilson T. (ed) 1990 Confocal Microscopy (London: Academic).
- Wilson T. and Sheppard C. J. R. 1984 Theory and Practice of Scanning Optical Microscopy (London: Academic).