Cutting-edge AFM-RAMAN-SICM System for Biological Studies
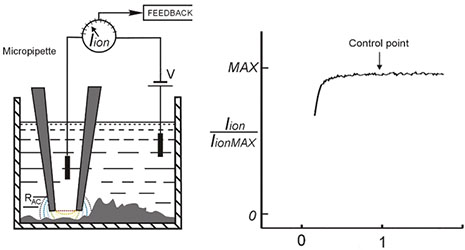
SICM (Scanning Ion Conductance Microscopy) is an SPM technique which uses nano-pipette (sharp glass electrode) for non-contact 3D surface mapping at high resolution. In SICM, the probe to sample distance is controlled via the decrease of ionic current flowing through the tip, as it approaches the sample surface.
Biophys.Journ. 73, 653-658
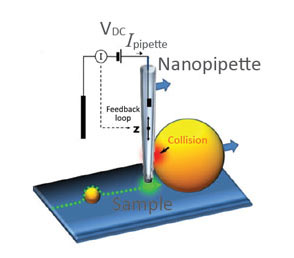
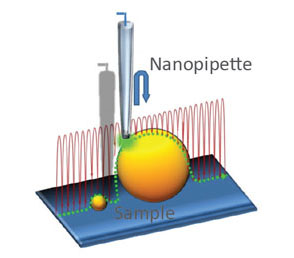
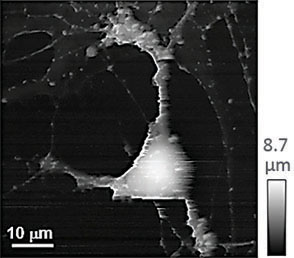
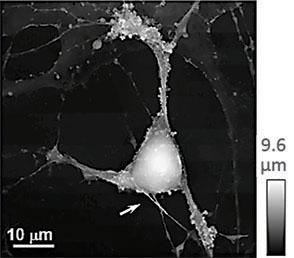
(a) Illustration of a scanning nanopipette probe operating in continuous scan mode colliding with a spherical object possessing a steep vertical slope. (b) Illustration of the hopping mode used in HPICM showing how the pipette is withdrawn to a position well above the sample before approaching the surface. (c,d) Topographical images of the same fixed hippocampal neuron obtained first with hopping mode (d) and then with continuous left-to-right raster scan mode (c), using the same nanopipette.
Hopping mode algorithm applied to SICM allows to image uneven and convoluted samples at high resolution ensuring that pipette always approaches from above rather than “dragging” along the surface. Nature Meth. (2009) 6: 279-281
Noncontact algorithm of hopping SICM enables stable, fast and high-resolution imaging of soft and highly corrugated objects like living cells under physiologically relevant conditions.
Scanning method ensures that the probe is always approaching the sample in vertical direction, thus, it becomes possible to visualize even those objects that are “suspended” in space.
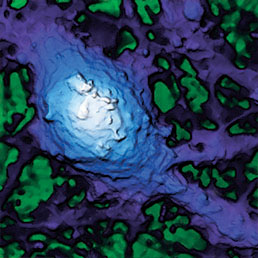
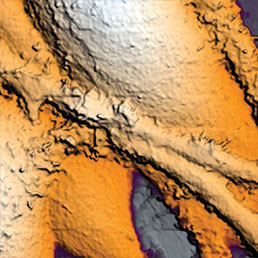
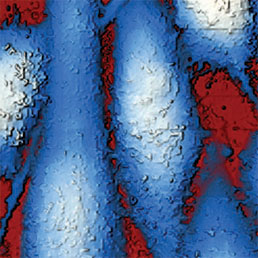
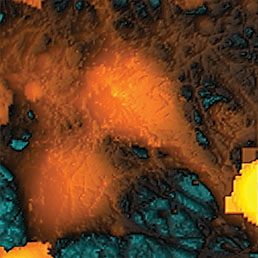
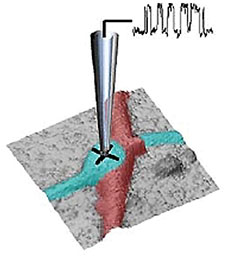
Extracellular pH mapping of living cancer cells with high spatial resolution and sensitivity can be implemented by means of SICM utilizing doublebarrel nanopipette. Morphology and pH-map of low-buffered living melanoma cells are shown from the left. Scale bars represent 20 μm.
Nature Comm. (2019) 10, 5610

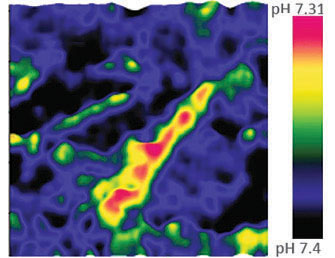
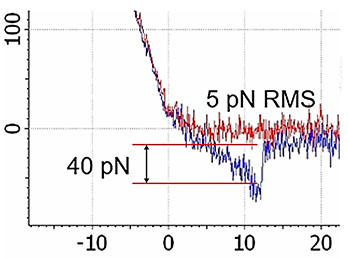
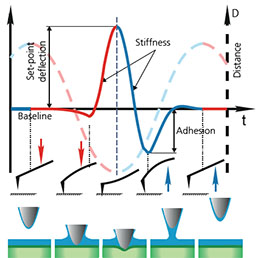
High-resolution AFM microscopy is available by means of Contact, Tapping and HybriD modes and is empowered by lowest signal-tonoise ratio of OBD loop on the market down to 25 fm/√Hz.
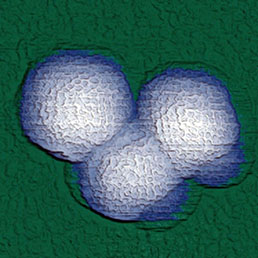
Combination of SICM and HybriD Mode™ AFM expands the boundaries of real-time quantitative nanomechanical mapping to 10 orders of elastic modulus keeping the possibility of single-point force spectroscopy experiments. Low- or noninvasive nature of tip-sample interaction allows to study delicate biological and jelly samples that are weakly attached to the substrate.
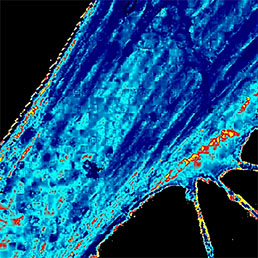
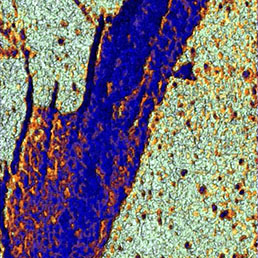
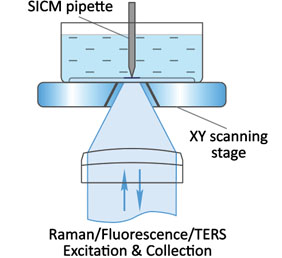
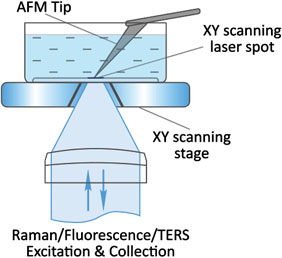
Flawless hardware and software integration of AFM/SICM with confocal Raman/fluorescence microscopy provides the widest range of additional information about the sample. Simultaneously measured AFM/SICM and Raman/fluorescence maps of exactly the same sample area deliver complementary information about sample physical properties and chemical composition.
When equipped with specially prepared probes, which are working as “nanoantennas”, AFM/SICM-Raman combination allows to perform optical mapping with resolution less than diffraction limit and is called TERS: Tip Enhanced Raman Scattering.